Back to 123a - Back to content
Records of some copepods. (Papa and Hołyńska, 2013).
CYCLOPOIDA, BILLBERG, 1820 (order)
An important group with at least 18 species recorded -
11 of these found in limnetic zone (Papa, R. D. S.,
M. K. Hołyńska, 2013). Anterior part of the body only a
little bigger than and less than twice the length of
the abdomen. First antenna short, with 6-17 segments,
never reaching the abdomen. Fifth leg not asymmetrical.
With two brood sacks.
Micro-predators, which feeds on protists, small
invertebrates and even small fish-larvae. Most copepods
have mixed diets though some tend to be carnivorous
while other predominantly are herbivorous.
General small-sized animals, able to avoid being
predated upon by fish (Tropocyclops and Thermocyclops).
(Alekseev, 2002a). The use of cyclopoid copepods
(Mesocyclops and at least one Thermocyclops species,
as potential biological control agents of
Dengue-carrying mosquitoes (Aedes spp.) has been
investigated by Ueda and Reid (2003a)
CYCLOPIDAE, RAFINESQUE, 1815 [BILLBERG, 1820] (family)
EUCYCLOPINAE, KIEFER, 1927
Last segment of fifth leg of female with 3
appendages. Caudal branches often with bristles.
Feed by seizing food when colliding with it.
Feed on bacteria, algae and zooplankton.EUCYCLOPS, CLAUS 1893.
CFifth leg articulated with the fifth thoracic segment,
angular in outline. First antenna with 6-12 segments.
Outer margin of caudal branches with small distinct
saw-like spines. Caudal branches usually very divergent
and 4-9 times as long as wide. Lateral spine near the end
of the caudal branch. The internal angle of the basipod
of 4th leg sharply produced. Both nauplii and copepodite
feed on algae (diatoms and filamentous green algae and
cyanobacteria (blue-green algae)). Littoral.
The geographical range of E. serrulatus extends from
North Africa, the Mediterranean basin, continental
Europe and Russia to most of Siberia and perhaps
Central Asia. In South-east Asia we know of
serrulatus-like specimens close to genotype A of the
type population (Northern Thailand and Southern China,
Guangdong Province)(ALEKSEEV et al. 2005).
124 a. Rami 4-5 times longer than wide. Outer margin with
dense row of small, clearly discernible saw-like small
spines. 5th leg with knife-like inner spine and two
setae; outer seta equal in length to spine, middle
seta about 1.3–1.5 times as long as spine. Egg sacs
rather divergent, compact and pointed at the end.
Size female 800-1450 μm. Littoral and tychoplanktonic,
e.g. rare in plankton samples. Found abundant in the
floodwater irrigating rice-fields (Schoenly 1998b).
Also in brackish water. Common.
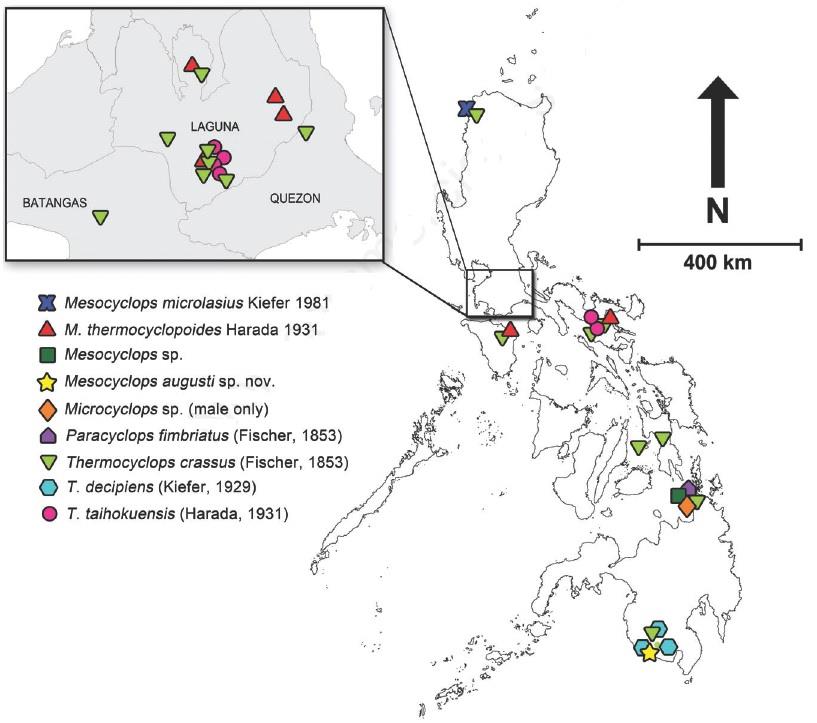
(Figures 124a1, 124a2)
- Laguna de Bay (nauplii and female), Paoay Lake,
La Mesa Dam, Taal Lake, Naujan, Lake Lanao, Taal lake.
Eucyclops serrulatus "type A", (FISHER, 1851)
[1978a+b, 1980, 1984a, 1986a, 1998b,Note: Some "candidate records" from the region:
Rami 4-5 times longer than wide.
- Not recorded from the Philippines. Distributed in Africa and Asia. Freshwater.
Eucyclops agiloides (SARS, 1909) (?)
Rami 5 times longer than wide
- Not recorded from the Philippines. Distributed in Africa and Asia. Fresh waters.
Eucyclops euacanthus (G. O. SARS, 1909)Rami rather long. 7 times longer than wide.
- Not recorded from the Philippines. Distribution: cosmopolitan, except Australia; fresh waters.Eucyclops macruroides denticulatus (GRAETER, 1903)
Rami very long. 9 times longer than wide.
- Not recorded from the Philippines. Distribution: Cosmopolitan, except Australia; fresh waters.
Eucyclops macrurus (G. O. SARS. 1863).
- Not recorded from the Philippines. Distributed in Asia: India and Java. Fresh waters.
Eucyclops permixtus KIEFER, 1928
E. serrulatus Female from Laguna de Bay
(Photo Rey Donne S. Papa, 2007)
TROPOCYCLOPS, KIEFER, 1927
Fifth leg articulated with the fifth thoracic segment,
angular in outline. First antenna with 6-12 segments.
Outer margin of caudal branches without such spines,
and only diverging slightly, 2 1/2 times as long as
wide. The internal angle of the basipod of 4th leg
rounded producedLittoral.
125 a. One species. Egg sacs closely opposed to abdomen.
Lateral spine in the middle of caudal branch.
Swimming rather than creeping form, swims on its back.
An especially small cyclopoid copepod. Usually size of
females 680-750 μm. Herbivorous and carnivorous.
Under in situ food conditions, T. p. mexicanus depended to
a larger extent on algae than invertebrate prey.
Daily mass-specific uptake rates for algae ranged
between 10 and 24% of its body mass versus 0.7–7% for
invertebrate prey. However, under enriched food
availability, T. p. mexicanus is able to ingest a biomass
equivalent to its body mass, with an algae (54%) and
prey (40%).Body size appears to be an important factor
for the relative importance of algal versus invertebrate
prey for cyclopoid copepods. (Adrian and Frost, M. 1992).
In ponds and littoral zone in lakes, tychoplanktonic and
planktonic (form pelagica). Rare. More than 10 subspecies
described in the tropics. One subspecies pantropic.
Genus needs revision. Records need confirmation
(Papa and Hołyńska 2013).
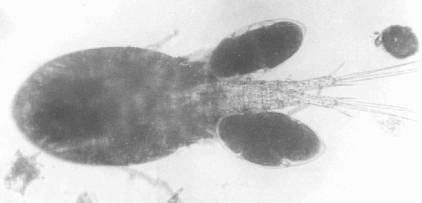
(Figures 125a1, 125a2)
- Laguna de Bay (female), La Mesa Dam, Lake Lanao, Paoay Lake.
Tropocyclops prasinus (FISHER, 1860)
(syn. Eucyclops prasinus)
[1978a+b, 1980, 1984a, 1986a, 2008b]
T. prasinus Female from Laguna de Bay. Preserved.
PARACYCLOPS, CLAUS, 1893
Fifth leg articulated with the fifth thoracic segment,
angular in outline. First antennawith 6-12 segments.
Anterior part of body clearly flattened dorsoventrally.
First antenna very short with 8 segments (6-11 worldwide),
reaching only to the middle of the cephalothorax.
Caudal branch long and thin, 5-6 times as long as wide,
dorsally with transverse row of small spines. Littoral.
126 a. Egg sacs closely opposed to abdomen, with few
large eggs. Adaptive species, eurytherm 13.5-39o Celsius in
alkaline water. Size female 860-900 μm., Benthic in lakes
and rivers. Also found in rice fields. Rare.
Older records need confirmation (Papa and Hołyńska 2013).
- Laguna de Bay (littoral, adult), Los Baños, Paoay Lake.
Paracyclops fimbriatus, (FISHER, 1853)
[1978a+b, 1980, 1986a, 2008b, 2013]
126 b. First antenna 11 segmented. Outer branch (exopodite) of
3rd leg with 3 spines. A predator of plant parasitic nematodes.
Cosmopolite.
See “Krepsdyr I ferskvann” (Crustacean In Freshwater):
http://www.nina.no/nb-no/milj%C3%B8overv%C3%A5king/krepsdyr.aspx
and (http://www.bar.gov.ph/database/barlib/items.asp?id=6231)
(Visited December 2014)
- Philippines
Paracyclops affinis (SARS, 1863)
[2001d]
ECTOCYCLOPS, BRADY, 1904
Fifth leg form a very short and wide plate without
articulation with the fifth (posterior) thoracic segment.
First antenna with 9-10 segments. Caudal branches short
and thick with several transverse rows of small spines.
More or less littoral.
One species recorded from the Philippines.
127 a. All dorsal spines of 5th leg more or less of equal length.
Egg sacs closely pressed to abdomen. Size of female
900-1000 μm. In all type of freshwater except running
water. Common.
Genus needs revision. Record need confirmation
(Papa and Hołyńska 2013).
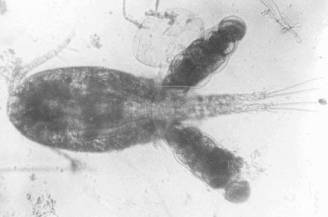
- Laguna de Bay (adult), Taal Lake, Naujan, Lake Lanao, Paoay Lake.
Ectocyclops phaleratus, (KOCK, 1838)
[1978a+b, 1980, 1984, 1986a, 2008a, 2011a]
E. phaleratus from Laguna de Bay
127 b. Inner dorsal spine of setae of 5th leg much longer than
the other two spines. Mainly benthic in lakes, marshes,
and ephemeral waters. Often considered as a subspecies
of E. phaleratus
- Not recorded in the Philippines. Distributed in
South-East Asia (Alekseev, 2002a)
Ectocyclops rubescens, (BRADY, 1904)
Note:
MACROCYCLOPS CLAUSE, 1893.
Is not recorded from the Philippines, but Mamaril & Fernando (1978b)
suggested that further sampling can reveal the presence of
M. distinctus (RICHARD, 1887) from Indonesia, Sri Lanka, India or
(Alekseev, 2002) the cosmopolitan species M. albidus (JURINE, 1820),
M. fuscus (JURINE. 1820) or the South-East Asian species M. neuter
(G. O. SARS, 1909)
To 128a